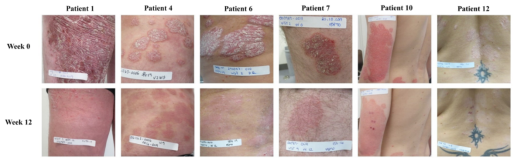
The executive director of the International Psoriasis Association and Professor Las Iversen’s team from Denmark published "HSP90 inhibitor RGRN-305 for oral treatment of plaque type psoriasis: efficacy, safety and biomarker results in an open-label proof-of-concept study " on British Journal of Dermatology (IF=9.3) on November 8th, 2021. The results showed that 6 of 11 patients responded to RGRN-305, with clinical symptom scores PASI (Psoriasis Area and Severity Index) improvement between 71% and 94%, and the drug showed an acceptable safety profile, especially in the low-dose group. RGRN-305 treatment significantly inhibited IL-23, TNF-α, and IL-17A signaling pathways and normalized histological changes and psoriatic lesion gene expression profiles in patients who responded to treatment. This study shows that HSP90 may be a new target for psoriasis treatment, and RGRN-305 is expected to be an innovative oral drug for psoriasis treatment based on a new mechanism of action.
论文链接:https://onlinelibrary.wiley.com/doi/10.1111/bjd.20880
Related News
- BEBT-503 Passes Phase I Clinical Trial in Australia, Marking a Milestone in BeBe...
- BeBetter Med’s Pan-mutant EGFR Inhibitor BEBT-109 Phase III Clinical Trial Appl...
- BeBetter Med’s First-in-Class New Drug BEBT-908 NDA Accepted
- BeBetter Med Inc. successfully holds the sponsors meeting & the first general me...
- BeBetter Med’s BEBT-209 Approved for Phase III Clinical Trials
- First-In-Class Drug RGRN-305 (BEBT-305) Clinical Proof-of-Concept Study Results ...
- First-In-Class Drug BEBT-908 Research Results of BEBT-908 Published on CANCER RE...
- BeBetter Med’s BEBT-908 granted Breakthrough Therapy designation


